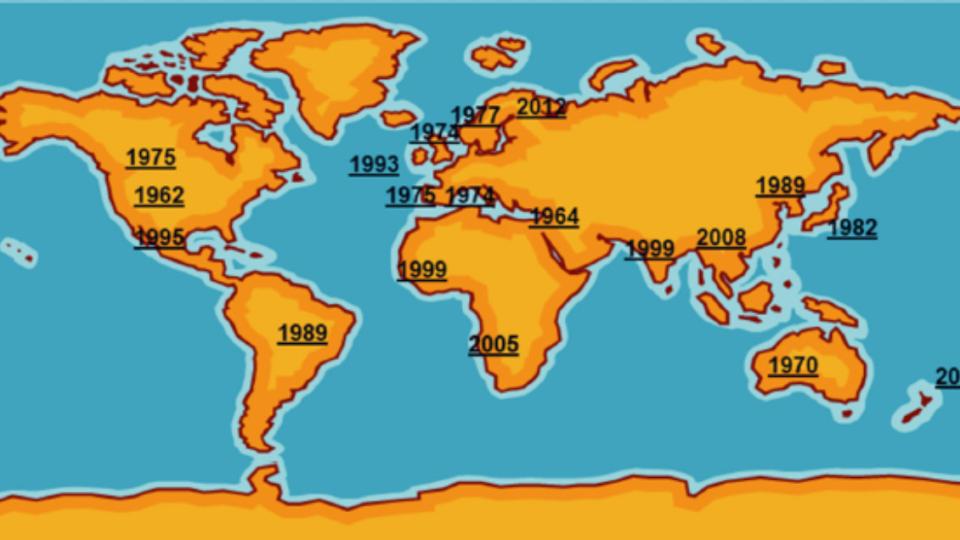
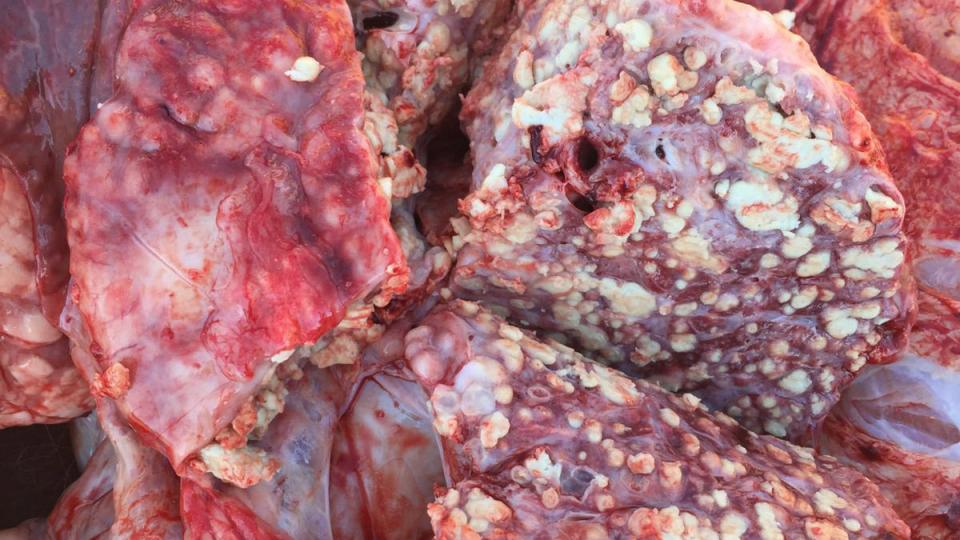
The current situation of M. bovis presents a difficult scenario to fight against this pathogen. The large antibiotic resistance to antimicrobial drugs frequently used in the field, added to the lack of an optimal vaccine, suggest that much more research is needed to obtain good control tools. In addition, the intrinsic characteristics of M. bovis, showing differences in virulence among field strains, the difficulty of diagnosis and the ability to become chronic or induce latent infections, make it a difficult pathogen to control. Considering that it is one of the main agents involved in BRD, good facility management, surveillance and control of other related pathogens is essential to minimize the impact of M. bovis on farms until new tools are developed.
Author: Carlos Montbrau, DVM PhD; Ester Taberner, DVM PhD; Ricard March.
References:
Aebi, M., van den Borne, B. H. P., Raemy, A., Steiner, A., Pilo, P., & Bodmer,M. (2015). Mycoplasma bovis infections in Swiss dairy cattle: A clinical investigation. Acta Veterinaria Scandinavica, 57, 10.
Askar H., Chen S., Hao H., Yan X., Ma L., Liu Y., Chu Y., (2021). Immune evasion of Mycoplasma bovis. Pathogens, 10, 297.
Bayoumi, F. A., Farver, T. B., Bushnell, B., & Oliveria, M. (1988). Enzootic mycoplasmal mastitis in a large dairy during an eight-year period. Journal of the American Veterinary Medical Association, 192, 905–909.
Biddle, M. K., Fox, L. K., & Hancock, D. D. (2003). Patterns of mycoplasma shedding in the milk of dairy cows with intramammary Mycoplasma infection. Journal of the American Veterinary Medical Association, 223, 1163–1166.
Brown, D. R., May, M., Bradbury, J. M., & Johansson, K.-E. (2015). Mollicutes. Bergey’s Manual of Systematics of Archaea and Bacteria. 1–8.
Castillo-Alcala, F., Bateman, K. G., Cai, H. Y., Schott, C. R., Parker, L., Clark, M. E., Caswell, J. L. (2012). Prevalence and genotype of Mycoplasma bovis in beef cattle after arrival at a feedlot. American Journal of Veterinary Research, 73, 1932–1943. https://doi.org/10.2460/ajvr.73.12.1932
Caswell, J. L., & Archambault, M. (2007). Mycoplasma bovis pneumonia in cattle. Animal Health Research Reviews, 2, 161–186. https://doi.org/10.1017/S1466252307001351
Cornelissen, J. B. W. J., de Bree, F. M., van der Wal, F. J., Kooi, E. A., Koene, M. G. J., Bossers, A., Wisselink, H. J. (2017). Mycoplasma detection by triplex real-time PCR in bronchoalveolar lavage fluid from bovine respiratory disease complex cases. BMC Veterinary Research, 13, 97. https://doi.org/10.1186/s12917-017-1023-6
Dudek K., Szacawa E., Nicholas R.A.J., (2021). Recent developments in vaccines for bovine mycoplasmoses caused by Mycoplasma bovis and Mycoplasma mycoides subsp. mycoides. Vaccines, 9, 549.
Dudek K., Nicholas R.A.J., Szacawa E., Bednarek D. (2020). Mycoplasma bovis infections – occurrence, diagnosis and control. Pathogens, 9, 640.
Gagea M.I., Bateman K.G., Shanahan R.A., van Dreumel T., McEwen B.J., Carman S., Archambault M., Caswell J.L., (2006). Naturraly ocurring Mycoplasma bovis – associated pneumonia and polyarthritis in feedlot beef calves. J Vet Diagn Invest 18, 29-40.
Houlihan, M. G., Veenstra, B., Christian, M. K., Nicholas, R., & Ayling, R. (2007). Mastitis and arthritis in two dairy herds caused by Mycoplasma bovis. Veterinary Record, 160, 126–127. https://doi.org/10.1136/vr.160.4.126
Justice-Allen, A., Trujillo, J., Corbett, R., Harding, R., Goodell, G., & Wilson, D. (2010). Survival and replication of Mycoplasma species in recycled bedding sand and association with mastitis on dairy farms in Utah. Journal of Dairy Science, 93, 192–202. https://doi.org/10.3168/jds.2009-2474
Kishimoto, M., Tsuchiaka, S., Rahpaya, S. S., Hasebe, A., Otsu, K., Sugimura, S., Kobayashi, S., Komatsu, N., Nagai, M., Omatsu, T., Naoi, Y., Sano, K., Okazaki-Terashima, S., Oba, M., Katayama, Y., Sato, R., Asai, T., Mizutani, T. (2017). Development of a one-run real-time PCR detection system for pathogens associated with bovine respiratory disease complex. Journal of Veterinary Medical Science, 79, 517–523. https://doi.org/10.1292/jvms.16-0489
Lysnyansky, I., & Ayling, R. D. (2016). Mycoplasma bovis: Mechanisms of resistance and trends in antimicrobial susceptibility. Frontiers in Microbiology, 27(7), 595.
Lysnyansky, I., Sachse, K., Rosenbusch, R., Levisohn, S., & Yogev, D. (1999). The vsp locus of Mycoplasma bovis: Gene organization and structural features. Journal of Bacteriology, 181, 5734–5741.
Maunsell F.P., Woolums A.R., Francoz D., Rosenbusch R.F., Step D.L., WIlson D.J., Janzen E.D., (2011). Mycoplasma bovis infections in cattle. J Vet Intern Med 25, 772-783.
Nicholas, R. A. J., Ayling, R. D., & Stipkovits, L. (2002). An experimental vaccine for calf pneumonia caused by Mycoplasma bovis. Vaccine, 20, 3569–3575. https://doi.org/10.1016/S0264-410X(02)00340-7
Nicholas, R. A. J., Ayling, R. D., & McAuliffe, L. (2008). Bovine respiratory disease. In R. Nicholas, R. Ayling & L. McAuliffe (Eds.), Mycoplasma diseases of ruminants (pp. 132–168), Oxfordshire: CABI Wallingford. https://doi.org/10.1079/9780851990125.0000
Nicholas, R. A. J., Fox, L. K., & Lysnyansky, I. (2016). Mycoplasma mastitis in cattle: To cull or not to cull. The Veterinary Journal, 216, 142–147. https://doi.org/10.1016/j.tvjl.2016.08.001
Radaelli, E., Luini, M., Loria, G. R., Nicholas, R. A. J., & Scanziani, E. (2008). Bacteriological, serological, pathological and immunohistochemical studies of Mycoplasma bovis respiratory infection in veal calves and adult cattle at slaughter. Research in Veterinary Science, 85, 282–290. https://doi.org/10.1016/j.rvsc.2007.11.012
Rosales, R. S., Puleio, R., Loria, G. R., Catania, S., & Nicholas, R. A. J. (2017). Mycoplasmas: Brain invaders? Research in Veterinary Science, 113, 56–61. https://doi.org/10.1016/j.rvsc.2017.09.006
Rosenbusch, R. F. (1994). Biology and taxonomy of the Mycoplasmas. In H. W. Whitford, R. F. Rosenbusch & L. H. Lauerman (Eds.), Mycoplasmosis in animals: Laboratory diagnosis (pp. 3–11). Ames, Iowa: Iowa State University Press.
Sachse K., Salam H.S.H., Diller R., Schubert E., Hoffmann B., Hotzel H. (2010). Use of a novel real-time PCR technique to monitor and quantitate Mycoplasma bovis in cattle herds with mastitis and respiratory disease. The Veterinary Journal 186, 299-303.
Timsit, E., Arcangioli, M. A., Bareille, N., Seegers, H., & Assi_e, S.(2012). Transmission dynamics of Mycoplasma bovis in newly received beef bulls at fattening operations. Journal of Veterinary Diagnostic Investigation, 24, 1172–1176. https://doi.org/10.1177/1040638712463211
Van der Merwe, J., Prysliak, T., & Perez-Casal, J. (2010). Invasion of bovine peripheral blood mononuclear cells and erythrocytes by Mycoplasma bovis. Infection and Immunity, 78, 4570–4578. https://doi.org/10.1128/IAI.00707-10
Wang, Y., Liu, S., Li, Y., Wang, Q., Shao, J., Chen, Y., & Xin, J. (2016). Mycoplasma bovis-derived lipid-associated membrane proteins activate IL-1b production through the NF-jB pathway via toll-like receptor2 and MyD88. Developmental and Comparative Immunology, 55,111–118.
Wilson, D. J., Skirpstunas, R. T., Trujillo, J. D., Cavender, K. B., Bagley, C. V., & Harding, R. L. (2007). Unusual history and initial clinical signs of Mycoplasma bovis mastitis and arthritis in first-lactation cows in a closed commercial dairy herd. Journal of the American Veterinary Medical Association, 230, 1519–1523. https://doi.org/10.2460/javma.230.10.1519
Wrathall, A. E., Ayling, R. D., & Simmons, H. (2007). Risks of transmitting mycoplasmas by semen and embryo transfer techniques in cattle, sheep, goats and pigs. CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources, 2(36), 1–31.