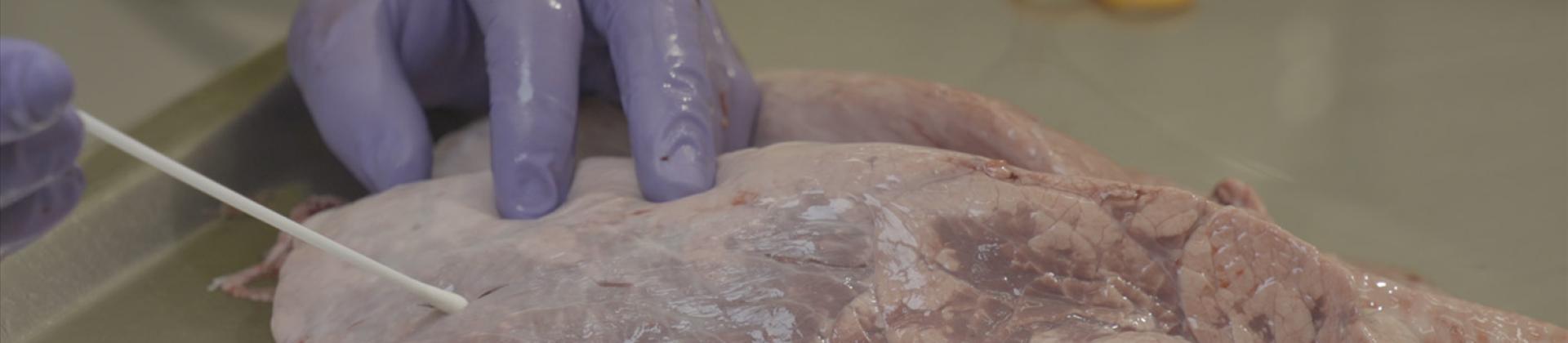
Mannheimia heamolityca is typically associated with fatal pneumonia13. Calves usually become infected immediately after birth, due to nasal contact with their mothers. In healthy animals, Mannheimia behaves as a commensal, until stressful factors appear. BRD prevention should not be based on regular antibiotic use but on welfare and management prevention practices, such as vaccination, in order to control this costly disease in cattle.
Author: Wojciech Ptak, DVM .
References:
1. C. Blakebrough-Hall, A. Dona, M. J. D’occhio, J. McMeniman & L. A González, 2020. Diagnosis of Bovine Respiratory Disease in feedlot cattle using blood 1H NMR metabolomics. Sci Rep 10:115
2. Edouard Timsit, DVM, PhDa, Chris McMullen, BScb, Samat Amat, MSc, PhDb,c,d, , Trevor W. Alexander, PhDc, 2020. Respiratory Bacterial Microbiota in Cattle From Development to Modulation to Enhance Respiratory Health Vet Clin North Am Food Anim 36, 297–320.
3. Griffin, D., Chengappa, M.M., Kuszak, J., McVey, D.S., 2010. Bacterial pathogens of the bovine respiratory disease complex. Vet Clin North Am Food Anim Pract 26, 381-394.
4. Roger J. Panciera, Anthony W. Confer. Pathogenesis and Pathology of Bovine Pneumonia Vet Clin North Am Food Anim Pract. 2010 Jul; 26(2): 191–214.
5. Jeyaseelan, S., Sreevatsan, S., Maheswaran, S.K., 2002. Role of Mannheimia haemolytica leukotoxin in the pathogenesis of bovine pneumonic pasteurellosis. Anim Health Res Rev 3, 69-82.
6. Mosier, D., 2015. Review of BRD pathogenesis: The old and the new. Animal Health Research Reviews, 15(2), 166-168.
7. Malazdrewich C, Thumbikat P, Maheswaran SK, 2004. Protective effect of dexamethasone in experimental bovine pneumonic mannheimiosis. Microbial Pathogenesis, 36(4), 227-236.
8. Zecchinon, L., Fett, T., Desmecht, D., 2005. How Mannheimia haemolytica defeats host defence through a kiss of death mechanism. Vet Res 36, 133-156.
9. Auad J, Carbonero Martínez A, Victoria Maure MV, Daffner JF, Gleser HD, 2001. Inoculation trial and humoral response with an inactivated oily vaccine against the bovine respiratory complex (BRC). Veterinaria Argentina, 18(176), 430-439.
10. Stevens RD, Dinsmore RP, Ellis RP, Katsampas M, 1997. Morbidity and mortality in young Holstein heifer calves vaccinated with a P. haemolytica leukotoxoid. Large Animal Practice, 18(6):23-29.
11. Tobias Oppermann, Nadine Busse, Peter Czermak, 2017. Mannheimia haemolytica growth and leukotoxin production for vaccine manufacturing — A bioprocess review. Electronic Journal of Biotechnology, 28, 95-100.
12. Rice, J. A., Carrasco-Medina, L., Hodgins, D. C., Shewen, P. E., 2007. Mannheimia haemolytica and bovine respiratory disease. Animal Health Research Reviews, 8(2), 117-128.
13. Laëtitia Dorso, Maud Rouault, Claire Barbotin, Christophe Chartier, Sébastien Assié, 2021. Infectious Bovine Respiratory Diseases in Adult Cattle: An Extensive Necropsic and Etiological Study. Animals (Basel). 2021 Aug; 11(8): 2280.