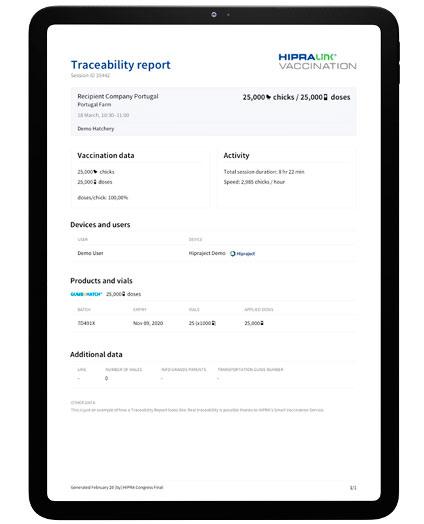
Furthermore, HIPRAlink® Vaccination, the third element of the SMART VACCINATION concept, has also been adapted to record all the vaccination data generated by Hipraject®, providing traceability and control for the entire vaccination process. It also helps veterinarians and hatchery managers to manage, analyse and certify their own vaccination activity:
· Management: Veterinarians or Hatchery Managers can easily check the GUMBOHATCH® vaccination data for each session: date and time, number of doses, number of chicks, information on the product, speed of the session, farm etc. The vaccination data are recorded automatically and are objective, transparent and reliable.
· Analysis: HIPRAlink® Vaccination can generate vaccination reports for GUMBOHATCH® to facilitate the analysis of data. In addition, the App shows statistical data in graph form (e.g. trend in the number of doses).
· Traceability: The user is able to generate traceability reports for GUMBOHATCH® that can be shared directly with the recipient company via HIPRAlink® Vaccination.