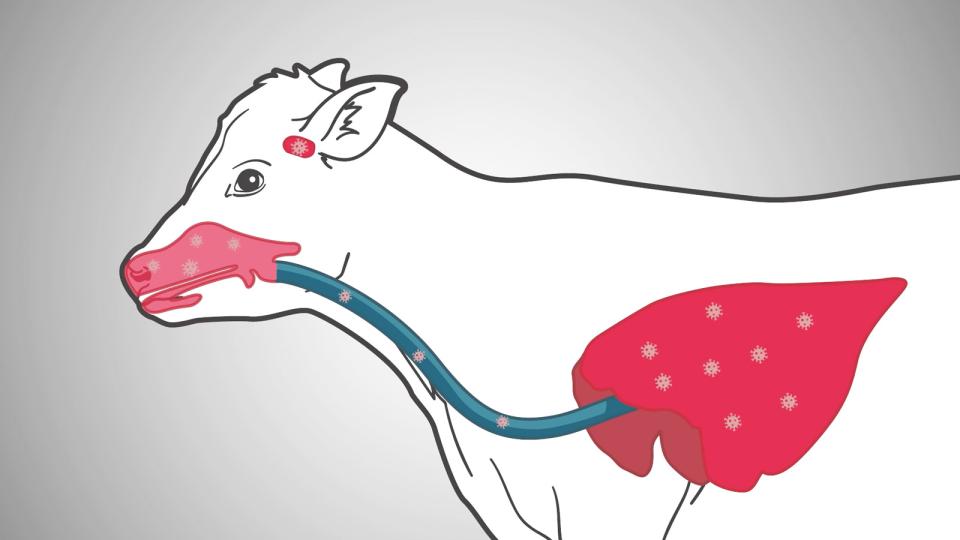
IBR remains a significant contributor to bovine respiratory disease complex and has significant effects on production and fertility in cattle herds. Its classical respiratory syndrome with involvement of the conjunctiva and the upper respiratory tract remains instantly recognisable and the highly infectious nature of the virus makes control in the face of an outbreak challenging. The typical herpesvirus facet of latency has made its eradication extremely difficult in most countries with only a handful of European nations making headway with eradication programs however control is possible with robust, 6 monthly vaccination protocols and monitoring.
Author: Oliver Maxwell BVSc BSc(Hons) MVM DipECBHM. Royal College Recognised Specialist in Cattle Health and Production. European Specialist in Bovine Health Management
References:
Ampe B., Duchateau L., Speybroeck N., Berkvens D., Dupont A., Kerkhofs P., Thiry E., Dispas M., 2012.Assessment of the long-term effect of vaccination on transmission of infectious bovine rhinotracheitis virus in cattle herds hyperimmunized with glycoprotein E–deleted marker vaccine American journal of veterinary research, 73(11), pp.1787-1793.
Brar, J.S., Johnson, D.W., Muscoplat, C.C., Shope Jr, R.E. and Meiske, J.C., 1978. Maternal immunity to infectious bovine rhinotracheitis and bovine viral diarrhea viruses: duration and effect on vaccination in young calves. American journal of veterinary research, 39(2), pp.241-244.
Bosch J.C., De Jong M.C.M., Franken P., Frankenas K., Hage J.J., Kaashoek M.J., Maris-Veldhuis M.A., Noordhuizen J.P.T.M., Van der PoeI W.H.M., Verhoeff J., Weerdmeester K., Zimmer G.M., Van Oirschot J.T., 1998 An inactivated gE negative marker vaccine and an experimental gD-subun:it vaccine reduce the incidence of bovine herpesvirus 1infections in the field. Vaccine, 16, pp.265-271.
Carter, J.J., Weinberg, A.D., Pollard, A., Reeves, R., Magnuson, J.A. and Magnuson, N.S., 1989. Inhibition of T-lymphocyte mitogenic responses and effects on cell functions by bovine herpesvirus 1. Journal of Virology, 63(4), pp.1525-1530.
D’arce, R.C.F., Almeida, R.S., Silva, T.C., Franco, A.C., Spilki, F., Roehe, P.M. and Arns, C.W., 2002. Restriction endonuclease and monoclonal antibody analysis of Brazilian isolates of bovine herpesviruses types 1 and 5. Veterinary microbiology, 88(4), pp.315-324.
Davies, D.H. and Carmichael, L.E., 1973. Role of cell-mediated immunity in the recovery of cattle from primary and recurrent infections with infectious bovine rhinotracheitis virus. Infection and Immunity, 8(4), pp.510-518.
Griebel, P.J., Ohmann, H.B., Lawman, M.J.P. and Babiuk, L.A., 1990. The interaction between bovine herpesvirus type 1 and activated bovine T lymphocytes. Journal of General Virology, 71(2), pp.369-377.
Griebel, P.J., Qualtiere, L., Davis, W.C., Lawman, M.J. and Babiul, L.A., 1987. Bovine peripheral blood leukocyte subpopulation dynamics following a primary bovine herpesvirus-1 infection. Viral immunology, 1(4), pp.267-286.
Jones, C., 2003. Herpes simplex virus type 1 and bovine herpesvirus 1 latency. Clinical microbiology reviews, 16(1), pp.79-95.
Mars M.H., de Jong M.C.M., Franken P., van Oirschot J.T., 2001.Efficacy of a live glycoprotein E-negative bovine herpesvirus 1 vaccine in cattle in the field. Vaccine, 19 (15-16), pp.1924-1930.
Muylkens, B., Thiry, J., Kirten, P., Schynts, F. and Thiry, E., 2007. Bovine herpesvirus 1 infection and infectious bovine rhinotracheitis. Veterinary research, 38(2), pp.181-209.
Pastoret, P.P., Aguilar-Setien, A., Burtonboy, G., Mager, J., Jetteur, P. and Schoenaers, F., 1979. The effect of repeated treatment with dexamethasone on the re-excretion pattern of infectious bovine rhinotracheitis virus and humoral immune response. Veterinary Microbiology, 4(2), pp.149-155.
Petrini S, Iscaro C, Righi C. Antibody Responses to Bovine Alphaherpesvirus 1 (BoHV-1) in Passively Immunized Calves., 2019. Viruses, 11(1) 23.
Spilki, F.R., Esteves, P.A., Lima, M.D., Franco, A.C., Chiminazzo, C., Flores, E.F., Weiblen, R., Driemeier, D. and Roehe, P.M., 2004. Comparative pathogenicity of bovine herpesvirus 1 (BHV-1) subtypes 1 (BHV-1.1) and 2a (BHV-1.2 a). Pesquisa Veterinária Brasileira, 24(1), pp.43-49.
Stabel, J.R., Spears, J.W. and Brown Jr, T.T., 1993. Effect of copper deficiency on tissue, blood characteristics, and immune function of calves challenged with infectious bovine rhinotracheitis virus and Pasteurella hemolytica. Journal of animal science, 71(5), pp.1247-1255.
Vannie, P., Capua, I., Le Potier, M. F., Mackay, D. K., Muylkens, B., Parida, S., Paton, D. J., & Thiry, E., 2007. Marker vaccines and the impact of their use on diagnosis and prophylactic measures. Revue scientifique et technique (International Office of Epizootics), 26(2), 351–372.
Wentink, G.H., Van Oirschot, J.T. and Verhoeff, J., 1993. Risk of infection with bovine herpes virus 1 (BHV1): a review. Veterinary Quarterly, 15(1), pp.30-33.
Winkler, M.T.C., Doster, A. and Jones, C., 1999. Bovine herpesvirus 1 can infect CD4+ T lymphocytes and induce programmed cell death during acute infection of cattle. Journal of virology, 73(10), pp.8657-8668.