The Bovine Respiratory Disease Complex (BRDC) is a huge problem worldwide. Cost estimations reach 3 billion dollars every year for the cattle industry1. Among the pathogens implicated in the complex the Bovine Respiratory Syncytial Virus (BRSV) has been associated with 45% of BRDC cases2 (and 60% in case of enzootic calf pneumonia3). These simple numbers show by themselves the tremendous importance of BRSV in cattle production.

Acute disease
Overview
The acute harmful effects of a virulent strain of BRSV circulating among young calves can be obvious. They are usually noticed in the group of calves aged between 1 and 6 months (yet, it is noteworthy that the age is not protecting by itself. If cattle are immunologically naive, they might die of the consequences of the infection at any age4). Those effects appear as a severe contagious respiratory disease: the morbidity can reach 80% of calves and mortality 20%5. In practice, even the most aggressive therapy is not successful in treating the most affected animals.
BRSV specificity
Such a life-threatening acute disease, frequently seen with BRSV, is rarely observed with PI-3. However, these two viruses induce necrosis of epithelial cells of bronchioles. Thus, the origin of the lethal consequences of BRSV infection cannot be found in its direct cytopathic effect but in the ability of the virus to trigger a severe acute inflammation in the whole organ.
Lesions
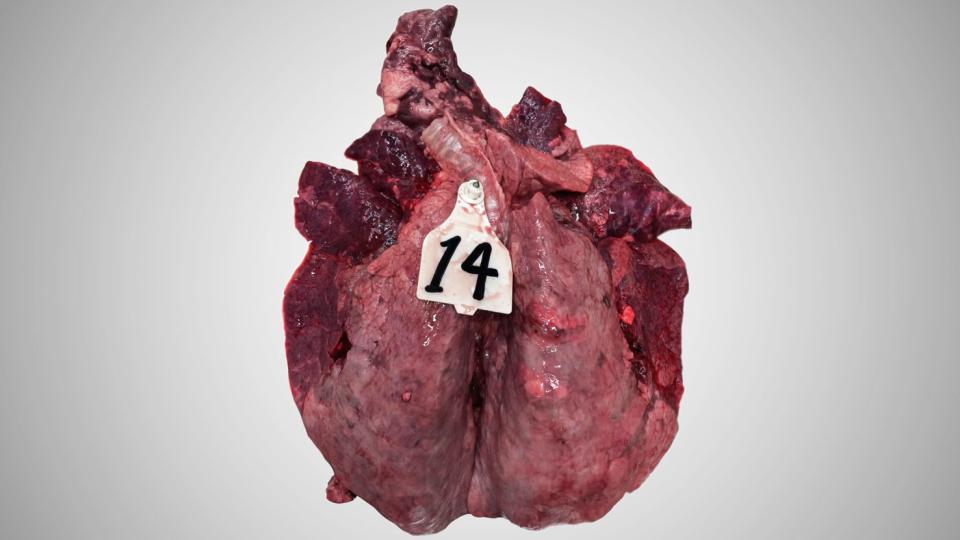
Macroscopically, this inflammation shows the hallmarks of an acute bronchointerstitial pneumonia with emphysema.
The lungs of calves that died after an acute BRSV infection are highly congestive. The congestion is irregular giving a heterogenous aspect to the parenchyma. In most cases, the antero-ventral region of the lungs is the most congestive and show some atelectatic portions. The lymph node reaction is always present and is usually obvious as the lymph nodes swelling can exceed 10 times the normal size. The emphysema also is characteristic of the disease.
The histopathological exams reveal a clear damage of bronchioles: epithelial cells necrosis, the presence of large multinucleated syncytial cells (that gave its name to the virus), and a mixed infiltration of neutrophils and some eosinophils (unusual in classical viral lesions). The epithelial necrosis can also involve the pneumocytes. As a consequence, reepithelialisation of the alveolar wall by type 2 pneumocytes is often present.
Pathogenicity
Although there are still lots of questions about the pathogenicity of the disease, the literature focusing on the disease caused by Pneumovirus (the human and the bovine syncytial virus) indicates that the worst clinical evolutions are induced by a Th2 biased immunoreaction5. That kind of reactions are close to type I hypersensitivity and implicate the action of the IgE, the eosinophils and sometimes the neutrophils6. In calves, the neutrophils seem to play a key role in this pathogeny7. Why, in the same herd, some animals trigger this biased deleterious inflammatory reaction and some other not, remains a tough question. The answer is dependent on the host-pathogen relationship.
· On the one hand, there are evidences that some virus strains are better able to elicit a severe inflammation. One small peptide was identified for BRSV to be one of the key players of the biased reaction: the virokinin8.. It has proven to be able to attract eosinophils (pivotal cells in Th2 reaction) and to contract smooth muscles of the bronchi. This last effect is probably the explanation of the severe emphysema that is characteristic of BRSV induced pulmonary lesions.
· On the other hand, in the pathogeny of the BRVS, the role of the host is not secondary. At field level, clinical BRSV outbreaks highlight the importance of the host. Indeed, unlike many other pathogens, which induce the most aggressive diseases in the weakest animals, BRSV often kills the healthiest animals. In beef farms, as an example, it is frequently seen that “the most beautiful” calves die first. This simple observation reflects the fact that a special configuration of the host’s organism has to be present to trigger the disease and not just a “classical” weakness.
Chronic evolution
The chronic consequences of the infection are far less obvious than the acute morbidity and mortality and are often underestimated.
Medium term consequences
The above-mentioned acute lesions reflect the action of the virus on the epithelial cells of bronchioles and alveoli. But, BRSV has also severe repercussions on the tracheal epithelial cells. The ciliated epithelium of the trachea, together with the goblet cells, form one of the most efficient innate defence of the respiratory system: the mucociliary escalator. In physiological conditions, the mucociliary escalator is a mechanical fence that prevent the colonization of the respiratory tract by Pasteurellacea, the main pathogenic bacterial family in cattle. Experimentations show that in healthy calves, 90% of the Pasteurella multocida experimentally introduced in the trachea are cleared within 4 hours. However, other studies point out that BRSV can decrease the mucociliary clearance by 50%9. In that conditions, the fence is a lot easier to cross for the nasopharyngeal pathogenic microflora. Moreover, the increased attraction of neutrophils10 during BRSV infection is thought to increase the effect of the Leukotoxin of Mannheimia haemolytica (the main virulence factor).
By its detrimental action on the tracheal epithelium, BRSV greatly promotes bacterial infections of the lungs.
Long term consequences
Because of the high level of exudation and tissue damage, the chronic long-term consequences of a bacterial infection are straightforward. For viral infections, the usual vision is an acute phase, that can be life-threatening, followed, if the animal survives, by a full recovery. This conception is not true with BRSV.
The best illustration of that is the case described by Klem and colleagues11. The authors measured the long-term effects of an outbreak of BRSV in an artificial insemination test centre. Because of the available zootechnic data in the centre, the imperceptible consequences of the infection became visible. Compared to the “non clinical/non treated” group, the bulls that showed symptoms had a constantly lower (4-10%) weight/age-ratio during the 8 month period of the study. But, more surprisingly, when the “non clinical/non treated” group was compared with a comparable group of the following year, this “healthy” group had a reduced daily gain of 111g/day.
This study shows how harmful is a clinical outbreak of BRSV for the productivity of the young herd. Even in asymptomatic animals, a virulent strain of BRSV can induce long-term lesions that will reduce cattle performances.
The origin of these long-term alterations is a lesion called bronchiolitis obliterans12. This is an unspecific lesion of the bronchioles which can be induced by BRSV in a very large number. It consists in small fibrous polypoid nodules that bulge in the lumen of the bronchioles causing a large obstruction. The functional consequence is a drop in the gas exchanges efficiency in the lungs.
Conclusion
Evidences of long-lasting deleterious consequences of BRSV outbreaks in naive animals (in addition to the obvious losses associated with the calves that die of the disease) are the reasons for which prevention is the only effective way to deal with this issue.
Author: Calixte Bayrou. Clinical Department of Production Animals, Faculty of Veterinary Medicine, University of Liège.
References:
1. Watts, J. L. & Sweeney, M. T. Antimicrobial resistance in bovine respiratory disease pathogens: measures, trends, and impact on efficacy. Vet. Clin. North Am. Food Anim. Pract. 26, 79–88, table of contents (2010).
2. Brodersen, B. W. Bovine Respiratory Syncytial Virus. Vet. Clin. North Am. Food Anim. Pract. 26, 323–333 (2010).
3. Meyer, G., Deplanche, M. & Schelcher, F. Human and bovine respiratory syncytial virus vaccine research and development. Comp. Immunol. Microbiol. Infect. Dis. 31, 191–225 (2008).
4. Valarcher, J.-F. & Taylor, G. Bovine respiratory syncytial virus infection. Vet. Res. 38, 153–180 (2007).
5. Gershwin, L. J. Bovine respiratory syncytial virus infection: immunopathogenic mechanisms. Anim. Health Res. Rev. 8, 207–213 (2007).
6. Tizard, I. R. Veterinary Immunology. (Elsevier Health Sciences, 2012).
7. Hägglund, S. et al. Proteome analysis of bronchoalveolar lavage from calves infected with bovine respiratory syncytial virus—Insights in pathogenesis and perspectives for new treatments. PLOS ONE 12, e0186594 (2017).
8. Valarcher, J.-F. Bovine respiratory syncytial virus lacking the virokinin or with a mutation in furin cleavage site RA(R/K)R109 induces less pulmonary inflammation without impeding the induction of protective immunity in calves. J. Gen. Virol. 87, 1659–1667 (2006).
9. Gershwin, L. J., Gunther, R. A., Hornof, W. J. & Larson, R. F. Effect of infection with bovine respiratory syncytial virus on pulmonary clearance of an inhaled antigen in calves. Am. J. Vet. Res. 69, 416–422 (2008).
10. McGill, J. L. & Sacco, R. E. ɣ∂T cells and the immune response to respiratory syncytial virus infection. Vet. Immunol. Immunopathol. 181, 24–29 (2016).
11. Klem, T. B., Kjæstad, H. P., Kummen, E., Holen, H. & Stokstad, M. Bovine respiratory syncytial virus outbreak reduced bulls’ weight gain and feed conversion for eight months in a Norwegian beef herd. Acta Vet. Scand. 58, (2016).
12. Jubb, Kennedy & Palmer’s Pathology of Domestic Animals: Volume 2. (Elsevier, 2016). doi:10.1016/C2012-0-00823-X